Research has played a vital role in changing the face of the medical field. From untreatable diseases to highly contagious diseases, research made wondrous achievements and solved the previously unsolvable. The research on HIV has also provided us with life-saving treatments like a prophylaxis from HIV. The WHO (World Health Organization) convened a meeting in June 2014 to develop guidelines for the use of HIV post-exposure prophylaxis (PEP). During this meeting, gaps in research were also uncovered. This paper published in Clinical Infectious Diseases® reports the background, methodology, results and conclusions of a study to create a framework for future research.
The Grading of Assessment, Evidence, Development and Evaluation (GRADE) system and clinical management pathway were used for formulating the research questions required for future research. The study reported and analyzed current WHO recommendations for HIV PEP and graded its quality of evidence as well. The recommendations were formulated with the help of the Guideline Development Group (GDG) created by the WHO.
For the study, a clinical management pathway for HIV PEP was made which included the following steps:
- Access to the PEP and its barriers
- Drug choice, timing and duration
- Adherence to PEP and strategies
- Follow-up and linkage to care
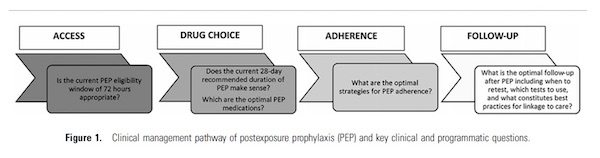
The formulated questions for each of the above mentioned clinical management pathway stage were thoroughly searched from current WHO guidelines and other online databases. The available data was collected, the gaps were identified and the GDG (Guidelines Development Group) helped the authors formulate future recommendations for each step.
Surprisingly, the study shows that the available guidelines of the WHO on the HIV PEP are mostly based on low-quality of evidence. Many of the clinical management questions that were formulated at the start of the study had no available study or recommendations.
On the whole, three study design formats for the future research were identified in the study which included the survey and interview driven research for identification of barriers related to PEP. Second was the establishment of a PEP registry on a global scale for drug choices, usage details, follow-ups and toxicities in specific drug regimen for PEP. Thirdly, randomized control trials for determination of the most authenticated evidence related to PEP.
For the study of access to PEP, the report suggests conducting cross sectional studies. To study the timing and duration of PEP, the recommendations are to have randomized control trials. These trials are also recommended for the study of the drug choice, adherence to the drug and the follow-up. Randomized control trials are lacking in comparing the HIV PEP drugs at different ages, hence the report recommends randomized control trials in it too.
The interesting thing reported in the study was that there is no future research needed in regard to the question whether a 28-day regimen for PEP is appropriate. The report mentioned that a 28 day PEP regimen is most appropriate and this recommendation does not need any future studies.

